According to the Renewable Energy Policy Network for the 21st Century (REN21), 85% of energy consumption in the United States relies on non-renewable energy sources that emit greenhouse gases such as CO2 into the atmosphere. The top source of renewable energy used in the United States comes from nuclear energy followed by wind, hydro, and solar. Considering that the use of most of these renewable sources is limited in residential and commercial sectors and the industrial and transportation sectors account for roughly 72% of energy consumption, it is essential to discover new renewable sources to reduce carbon emission. Hydrogen has been identified as a key source for reducing CO2 emissions with each passing decade as shown in the International Energy Agency (IEA) net zero by 2050 roadmap. Hydrogen can be produced through a variety of different pathways, such as electrolysis, photochemical, and thermochemical. Of these methods, solar thermochemical processes are especially promising because they only require solar energy with the heat and steam as the input. The material undergoes reduction and oxidation cycles to convert steam to hydrogen, which can then be captured for use.
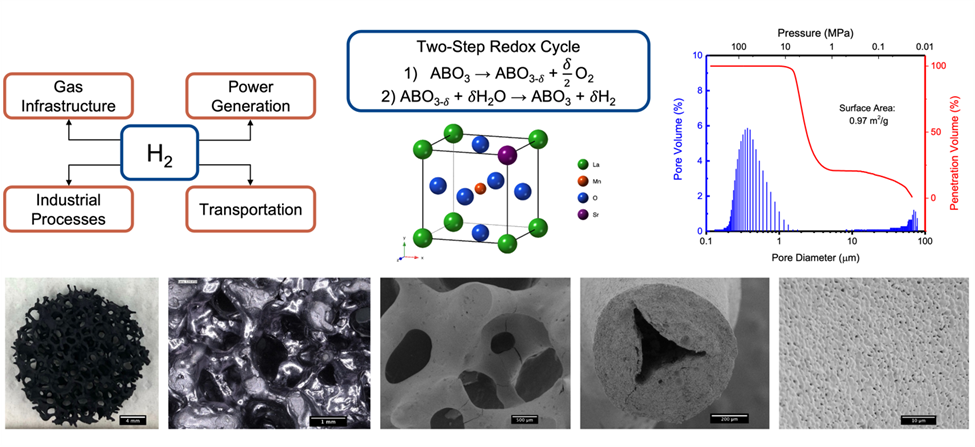
To increase hydrogen production by using the solar thermochemical cycle, we are synthesizing new materials as controlled porous foams. The large pores present in foams are ideal for hydrogen production because of their enhanced heat transfer capabilities compared to powders and pellets. Therefore, we use the replica method to fabricate highly porous foams with large mm-sized pores. The porosity of the foams is controlled through template selection and sintering profiles to include dual-scale porosity. Additionally, we control the rheology of the suspension for dip coating to ensure removal of the cell windows upon drying to obtain high open porosity in the foam. Beyond this, we also synthesize new material compositions by various synthesis methods including Pechini method and solid-state synthesis. These new compositions focus on perovskites and pyrochlores because they offer a high degree of tunability with many suitable A and B-site elements, substitutions, and dopants. We aim to understand the difference in redox performance for different compositions, and the effect of microstructural characteristics on the kinetic performance of the foams.
Currently we have highly porous (> 80%) lanthanum strontium manganite replica foams fabricated for testing in a water splitting furnace. The foams retained their large sized pores (D50 = 0.95 mm) and approximately 4% micron sized porosity (D50 = 0.4 m) in the struts after sintering. X-ray diffraction of the foams after 50 cycles at 1400°C reveal no detectable secondary phases in the bulk of the foam.